
Contact
- (210) 450-8157
- tumanov@uthscsa.edu
Department
Microbiology, Immunology & Molecular GeneticsTumanov, Alexei V., M.D., Ph.D.
Associate Professor
Personal Statement:
Dr. Alexei Tumanov’s research focuses on the regulation of mucosal immunity and cancer. We investigate how the immune system regulates the delicate balance between protective immunity and immunopathology at mucosal surfaces, particularly in the gut. The goal of Dr. Tumanov`s research program is to combine molecular data with in vivo models to understand the mechanisms underlying homeostatic and pathological conditions for the development of effective immunotherapies.
Dr. Tumanov graduated from Russian State Medical University, Moscow, Russia. He did his PhD work in the laboratory of Dr. Sergei Nedospasov at the Engelhardt Institute of Molecular Biology, Moscow, in collaboration with several laboratories in Europe and US, including NCI-Frederick (NIH), the Jackson Laboratory (Dr. Chervonsky`s lab), Institute of Experimental Immunology, Zurich (Dr. Zinkernagel`s lab). In 2004, Alexei joined laboratory of Dr. Yang-Xin Fu at the University of Chicago, Department of Pathology for postdoctoral training. In 2011, Alexei started his research lab at Trudeau Institute, NY. Dr. Tumanov was recruited to the Department of Microbiology, Immunology, and Molecular Genetics as an Associate Professor in Fall 2016.
Education
M.D., Russian State Medical University, Moscow
“Oncoimmunology” Educational Program in Immunology and Cancer Biology, Cancer Research Institute and Moscow State University (www.oncoimmunology.ru)
Ph.D., Molecular Biology, Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, Moscow
Research
We are interested in the regulation of mucosal immunity and cancer. Our main research is centered on understanding the biology of lymphotoxin (LT), member of tumor necrosis factor superfamily of cytokines. Although LT and its receptor, LTβR are known as key regulators of lymphoid organ development and maintenance, recent studies revealed the critical role of LT in protection against several mucosal pathogens and in regulation of intestinal inflammation. To understand the role of LT signaling in disease, we generated various mouse strains with genetically modified components of LT pathway. Specific ongoing projects include:
1) Understanding the role of lymphotoxin (LT) in inflammatory bowel disease and colorectal cancer.
Our recent studies reveal the critical role of LT in the regulation of innate lymphoid cells (ILCs) in the gut. ILCs are heterogeneous cell population which play key roles in the regulation of inflammation and immunity at mucosal surfaces, particularly in gastrointestinal tract. Despite recent advances in dissecting the roles of different ILC populations in intestinal homeostasis, the cellular and molecular mechanisms of protective versus pathogenic responses mediated by distinct populations of ILCs in inflammatory bowel disease (IBD) remain poorly understood, which limits the development of novel therapies. The goal of this project is to elucidate how LTβR signaling regulates intestinal inflammation in IBD and colitis-associated cancer, the major fatal complication for patients with IBD. We are also developing nanomaterial-based therapeutic approaches in intestinal injury.
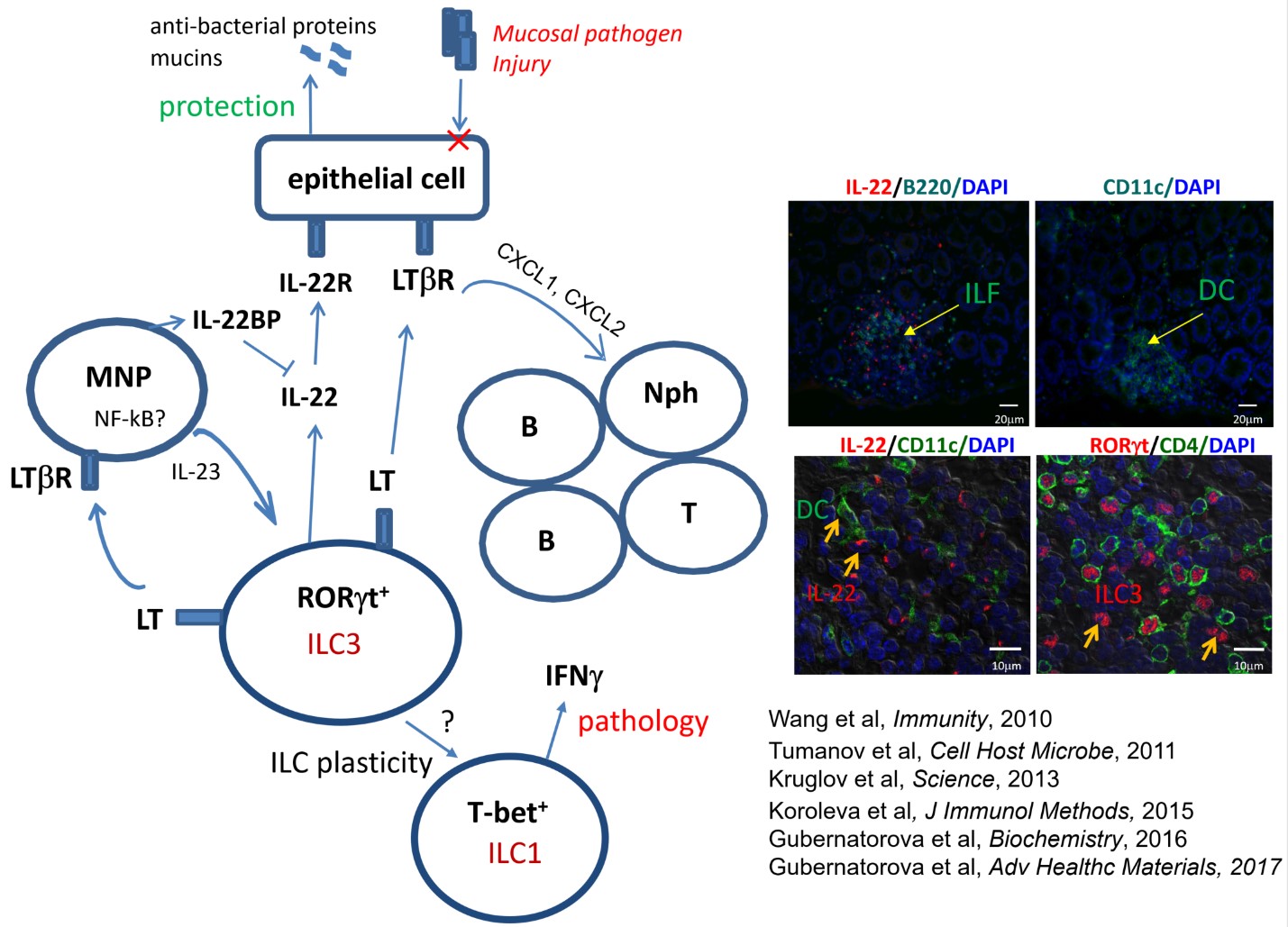
2) Investigating the role of innate lymphoid cells in Campylobacter-induced colitis.
Campylobacter jejuni is a common human enteric pathogen that causes acute enterocolitis and increases the risk of developing long-term intestinal dysfunction. Our data suggest that innate immune mechanisms contribute to intestinal pathology in C. jejuni-induced colitis. We investigate how distinct populations of ILCs regulate intestinal inflammation in C. jejuni-induced colitis.
3) Immune control of chronic pain.
Chronic pain is a significant health problem that has led to an epidemic of opioid analgesic overuse and overdose. Persistent inflammation is associated with chronic pain, however the cellular and molecular mechanisms remain poorly understood. The goal of this project is to define immune mechanisms controlling inflammatory- and chemotherapy-induced neuropathic pain.
4) Tumor immunotherapy.
Novel immunotherapies for cancer are having major clinical impact. However, accompanying immune-mediated adverse reactions, such as liver toxicity, remain the major obstacle of these therapies. The goal of this project is to improve effectiveness of cancer therapy by targeting LTβR pathway in the liver.
Awards & Accomplishments
- 1999 Research Training Fellowship of International Agency for Research on Cancer
- 2001 Outstanding Scholar Award. International Cytokine Society
- 2002 International Union Against Cancer (IUCC), Cancer Technology Transfer Fellowship
- 2004 Grant from Russian Foundation for Basic Research
- 2007 AAI and Keystone Conference Junior Faculty Travel Awards
- 2007 Scientist Development Grant, American Heart Association
- 2008 Pilot and Feasibility Award, University of Chicago Digestive Diseases Research Center
- 2010 Career Development Award, Crohn’s and Colitis Foundation of America
- 2012 CCFA Shanthi Sitaraman Memorial Young IBD Investigator Award
- 2013 Junior Faculty Travel Award, 14th International TNF Conference
- 2014 Biomedical Research Grant, American Lung Association
- 2014 Senior Research Award, Cohn’s and Colitis Foundation of America
- 2017 The Max and Minnie Tomerlin Voelcker Fund Investigator Award
- 2018 Peter Bradley Carlson Trust Award
Lab Members
Ekaterina Koroleva, Assistant Professor/Research
Amanda Munoz, Postdoctoral Fellow
Sergey Shein, Visiting Scientist
Ana Korchagina, Visiting Scientist
Jing Xi, M.D. Student
Shou Yajun, M.D. Student
Publications
- Muraoka WT, Korchagina AA, Xia Q, Shein A, Jing X., Lai Z., Weldon K.S., Wang L-J, Chen Y., Kummer L.W., Mohrs M., Vivier E., Koroleva EP, Tumanov A.V. Campylobacter infection promotes IFNγ-dependent intestinal pathology via ILC3 to ILC1 conversion. Mucosal Immunol. May;14(3):703-716, 2021. PMID 33214656.
- Vanderkerken M, Baptista AP, Giovanni M, Fukuyama S, Browaeys R, Scott CL, Norris PS, Eberl G, Di Santo JP, Vivier E, Saeys Y, Hammad H, Cyster JG, Ware CF, Tumanov AV*, De Trez C*, Lambrecht BN*. ILC3s control splenic cDC homeostasis via lymphotoxin signaling. J Exp Med. 2021, 218(5):e20190835. *Contributed equally. PMID: 33724364.
- Riffelmacher T, Giles DA, Zahner S, Dicker M, Andreyev AY, McArdle S, Perez-Jeldres T, van der Gracht E, Murray MP, Hartmann N, Tumanov AV, Kronenberg M. Metabolic activation and colitis pathogenesis is prevented by lymphotoxin β receptor expression in neutrophils. Mucosal Immunol. 2021, May;14(3):679-690. doi: 10.1038/s41385-021-00378-7. PMID 33214656.
- Jing X, Korchagina AA, Shein SA, Muraoka WT, Koroleva E, Tumanov AV. IL-23 contributes to Campylobacter jejuni-induced intestinal pathology via promoting IL-17 and IFNγ responses by innate lymphoid cells. Front Immunol 2021, Jan 6;11:579615. doi: 10.3389/fimmu.2020.579615. PMID: 33488580.
- Mecklenburg J, Zou Y, Wangzhou A, Garcia D, Lai Z, Tumanov AV, Dussor G, Price TJ, Akopian AN. Transcriptomic sex differences in sensory neuronal populations of mice. Sci Rep. 2020 Sep 17;10(1):15278. PMID: 32943709.
- Koprivsek JJ, He Y, Song C, Zhang N, Tumanov A.V., Zhong G. Evasion of innate lymphoid cell-regulated gamma interferon responses by chlamydia muridarum to achieve long-lasting colonization in mouse colon. Infect Immun 2020 Feb 20;88(3):e00798-19. doi: 10.1128/IAI.00798-19, PMID: 31818961.
- Gubernatorova E.O., Namakanova O.A., Tumanov A.V., Drutskaya M.S., Nedospasov S.A. Mouse models of severe asthma for evaluation of therapeutic cytokine targeting. Immunol Lett. 2018. 2019 Mar;207:73-83. PMID: 30659868.
- James K.D., Cosway E.J., Lucas B., White A.J., Parnell S.M., Carvalho-Gaspar M., Tumanov A.V., Anderson G., Jenkinson W.E. Endothelial cells act as gatekeepers for LTbR-dependent thymocyte emigration. J Exp Med, Nov 13. doi: 10.1084/jem.20181345, 2018. PMID: 30425120.
- Giles D.A., Zahner S., Krause P., van der Gracht E., Riffelmacher T., Morris V., Tumanov A.V., Kronenberg M. The tumor necrosis factor superfamily members TNFSF14 (LIGHT), lymphotoxin β and lymphotoxin β receptor interact to regulate intestinal inflammation. Front Immunol, doi: 10.3389/fimmu.2018.02585, 2018. PMID: 30524422.
- Gubernatorova E.O., Gorshkova E.A. Namakanova O.A., Zvartsev R.V., Hidalgo K, Drutskaya M.S., Tumanov A.V., Nedospasov S.A. Non-redundant functions of IL-6 produced by macrophages and dendritic cells in allergic airway inflammation. Front Immunol. doi: 10.3389/fimmu.2018.02718, 2018. PMID: 30534125.
- Behnke K., Zhuang Y., Xu H.C., Sundaram B., Reich M., Shinde P.V., Huang J., Modares N.F., Tumanov A.V., Polz R., Scheller J., Ware C.F., Pfeffer K., Keitel V., Häussinger D., Pandyra A.A., Lang K.S., Lang P.A. B cell-mediated maintenance of CD169+ cells is critical for liver regeneration. Hepatology, May 9. doi: 10.1002/hep.30088, 2018. PMID: 29742809.
- Bakshi S.F., Guz N., Zakharchenko A., Deng H., Tumanov A.V., Woodworth C.D., Minko S., Kolpashchikov D.M., Katz E. Nanoreactors based on DNAzyme-functionalized magnetic nanoparticles activated by magnetic field. Jan 18;10(3):1356-1365. doi: 10.1039/c7nr08581h. 2018. PMID: 29297526.
- Koroleva E.P., Fu Y.X., Tumanov A.V. Lymphotoxin in physiology of lymphoid tissues – Implication for antiviral defense. Cytokine. Jan;101:39-47. pii: S1043-4666(16)30472-0, 2018. PMID: 27623349.
- Schaeuble K, Britschgi M.R., Scarpellino L., Favre S., Xu Y., Koroleva E., Lissandrin T.K.A., Link A., Matloubian M., Ware C.F., Nedospasov S.A., Tumanov A.V., Cyster J.G., Luther S.A. Perivascular fibroblasts of the developing spleen act as LTα1β2-dependent precursors of both T and B zone organizer cells. Cell Rep. 2017 Nov 28, 21(9):2500-2514, 2017. PMID: 29186687.
- Cosway, E.J., Lucas B., James K.D., Parnell S.M., Carvalho-Gaspar M., White A.J., Tumanov A.V., Jenkinson W.E., Anderson G. Redefining thymus medulla specialization for central tolerance. J Exp Med. Nov 6, 214(11):3183-3195, 2017. PMID: 28830910.
- Bakshi S.F., Guz N., Zakharchenko A., Deng H., Tumanov A.V., Woodworth C.D., Minko S., Kolpashchikov D.M., Katz E. Magnetic field-activated sensing of mRNA in living cells. J Am Chem Soc. Sep 6, 139(35):12117-12120, 2017. PMID: 28817270.
- Zhang Y., Kim T-J, Wroblewska J.A., Tesic V., Upadhyay V, Weichselbaum R.R., Tumanov A.V., Tang H., Guo X., Tang H., Fu Y-X. ILC3-derived lymphotoxin prevents microbiota-dependent inflammation. Cell Mol Immunol, Jun 5. doi: 10.1038/cmi.2017.25, 2017. PMID: 28579615.
- Gubernatorova E.O., Liu X, Othman A., Muraoka W.T, Koroleva E.P., Andreescu S., Tumanov A.V. Europium-doped cerium oxide nanoparticles limit reactive oxygen species formation and ameliorate intestinal ischemia-reperfusion injury. Adv Healthc Mater, May 8. doi: 10.1002/adhm.201700176, 2017. PMID: 28481012.
- King I.L., Mohrs K., Meli A.P., Downey J., Lanthier P., Tzelepis F., Fritz J.H., Tumanov A.V., Divangahi M., Leadbetter E.A., Mohrs M. Intestinal helminth infection impacts the systemic distribution and function of the naive lymphocyte pool. Mucosal Immunol, Jan 25. doi: 10.1038/mi.2016.127, 2017. PMID: 28120841.
- Gubernatorova E.O. Tumanov A.V. Tumor necrosis factor and lymphotoxin in regulation of intestinal inflammation. Biochemistry (Mosc). 81 (11), 1309-1325, 2016. PMID: 27914457.
- Lucas B, James K.D., Cosway E.J., Parnell S.M., Tumanov A.V., Ware C.F., Jenkinson W.E., Anderson G. Lymphotoxin β receptor controls T cell progenitor entry to the thymus. J Immunol. 197(7):2665-72, 2016. PMID: 27549174.
- Gubernatorova E.O., Koroleva E.P., Halperin S., Perez-Chanona E., Jobin C., Tumanov A.V. Murine model of intestinal ischemia-reperfusion injury. J Vis Exp, May 11;(111). doi: 10.3791/53881, 2016. PMID: 27213580.
- Shaabani N., Khairnar V., Honke N., Duhan V., Zhou F., Tur R.F., Häussinger D., Recher M., Tumanov A.V., Hardt C., Pinschewer D., Chrisiten U., Lang P.A., Lang K.S. Two separate mechanisms of enforced viral replication balance innate and adaptive immune activation. J Autoimmun. Feb, 67:82-9, 2016. PMID: 26553386.
- Macho-Fernandez E., Koroleva E.P., Spencer C.M., Tighe M., Torrado E., Cooper A.M, Fu Y-X, Tumanov A.V. Lymphotoxin beta receptor signaling limits mucosal damage through driving IL-23 production by epithelial cells. Mucosal Immunol, Mar, 8(2):403-13, 2015. PMID: 25183367.
- Koroleva E.P., Halperin S., Gubernatorova E.O., Spencer C.M, Tumanov A.V. Citrobacter rodentium- induced colitis: a robust model to study mucosal immune responses in the gut. J Immunol Methods. 421:61-72, 2015. PMID: 25702536.
- Xu H.C., Huang J., Khairnar V., Duhan V., Pandyra A.A, Grusdat M., Shinde P., McIlwain D.R., Maney S.K., Gommerman J., Löhning M. Ohashi P.S., Mak T.W., Pieper K., Sic H., Speletas M., Eibel H., Ware C.F., Tumanov A.V., Kruglov A.A., Nedospasov S.A., Häusinger D., Recher M., Lang K.S., Lang P.A. Deficiency of the B cell-activating factor receptor results in limited CD169+ macrophage function during viral infection. J Virol. 89(9):4748-59, 2015. PMID: 25673724.
- Wolf M.J., Adili A., Piotrowitz K., Abdullah Z., Boege Y., Stemmer K., Ringelhan M., Simonavicius N., Egger M., Wohlleber D., Lorentzen A., Einer C., Schulz S., Clavel T., Protzer U., Thiele C., Zischka H., Moch H., Tschöp M., Tumanov A.V, Haller D., Unger K., Karin M., Kopf M., Knolle P., Weber A., Heikenwalder M. Metabolic activation of intrahepatic CD8(+) T Cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell, Oct 13;26(4):549-64, 2014. PMID: 25314080.
- Szaba F.M., Kummer L.W., Duso D.K., Koroleva E.P., Tumanov A.V., Cooper A.M., Bliska J.B., Smiley S.T., Lin J.S. TNFα and IFNγ but not perforin are critical for CD8 T cell-mediated protection against pulmonary Yersinia pestis infection. PLoS Pathog. 10(5):e1004142. doi: 10.1371/journal.ppat.1004142, 2014. PMID: 24854422.
- Kruglov A.A., Grivennikov S.I., Kuprash D.V., Winsauer C., Prepens S., Seleznik G. M., Heikenwalder M., Eberl G., Littman D.R., Tumanov A.V., Nedospasov S.A. Non-redundant function of soluble LTα3 produced by innate lymphoid cells in intestinal homeostasis. Science, 342(6163):1243-6, 2013. PMID: 24311691.
- Chellan B., Koroleva E.P., Sontag T.J., Tumanov A.V., Fu Y-X, Getz G.S., Reardon C.A.. LIGHT/TNFSR14 can regulate hepatic lipase expression by hepatocytes independent of T cells and Kupffer cells. PLoS One. 2013; 8(1): e54719. doi: 10.1371/journal.pone.0054719. PMID: 23355893.
- Upadhyay , Poroyko V., Kim T.J., Devkota S., Fu S., Liu D., Tumanov A.V., Koroleva E.P., Deng L., Nagler C., E. B. Chang, H. Tang, Y. X. Fu. Lymphotoxin regulates commensal responses to enable diet-induced obesity. Nat Immunol., 13(10): 947-53, 2012. PMID: 22922363.
- Moseman E. A., Iannacone M., Bosurgi L., Tonti E., Chevrier N., Tumanov A., Fu Y-X, N. Hacohen, von Andrian, U.H. 2012. B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity. Immunity, 36(3):415-26, 2012. PMID: 22386268.
- Tumanov AV, Koroleva EP, Guo X, Wang Y, Kruglov A, Nedospasov S, Fu YX. Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell Host Microbe, 10(1):44-53, 2011. PMID: 21767811.
- Kruglov AA, Tumanov AV, Grivennikov SI, Shebzukhov YV, Kuchmiy AA, Efimov GA, Drutskaya MS, Scheller J, Kuprash DV, Nedospasov SA. Modalities of experimental TNF blockade in vivo: mouse models. Adv Exp Med Biol.,691:421-31, 2011. PMID: 21153347.
- Daller B, Müsch W, Röhrl J, Tumanov AV, Nedospasov SA, Männel DN, Schneider-Brachert W, Hehlgans T. Lymphotoxin-β receptor activation by lymphotoxin-α(1)β(2) and LIGHT promotes tumor growth in an NFκB-dependent manner. Int J Cancer,128(6):1363-70, 2011. PMID: 20473944.
- Wang Y., Koroleva E.P., Kruglov A.A., Kuprash D.V., Nedospasov S.A., Fu Y-X, Tumanov A.V. Lymphotoxin beta receptor signaling in intestinal epithelial cells orchestrates innate immune responses against mucosal bacterial infection. Immunity, 32(3): 403-13, 2010. PMID: 20226692.
- Chen L., Park S-M., Tumanov A.V., Hau A., Sawada K., Feig C., Turner J.R., Fu Y-X., Romero I., Lengyel E., Peter M.E. CD95/FAS promotes tumor growth. Nature, 465(7297):492-6, 2010. PMID: 20505730.
- Tumanov A. V., Grivennikov S.I., Kruglov A.A., Shebzukhov Y.V., Koroleva EP, Piao Y., Cui X-Y., Littman D.R., Kuprash D.V. Nedospasov S.A. Cellular source and molecular form of TNF specify its distinct functions in organization of secondary lymphoid organs. Blood, 116 (18):3456-64, 2010. PMID: 20634375.
- Tumanov A.V., Koroleva E.P., Christiansen P.A., Khan M.A., Ruddy M.J., Burnette B., Papa S., Franzoso G., Nedospasov S., Fu Y-X., Anders R.A. T cell derived lymphotoxin regulates liver regeneration. Gastroenterology, 136(2):694-704, 2009. PMID: 18952083.
- Heikenwalder, M., Prinz, M., Zeller, N., Lang, K.S., Junt, T., Rossi, S., Tumanov, A., Schmidt, H., Priller, J., Flatz, L., Rülicke T, Macpherson A.J., Holländer G.A., Nedospasov S.A., Aguzzi A. Overexpression of lymphotoxin in T cells induces fulminant thymic involution. Am J Pathol. 172:1555-1570, 2008. PMID: 18483211.
- Liepinsh D.J., Kruglov A.A, Galimov A.R., Shakhov A.N., Shebzukhov Y.V., Kuchmiy A.A., Grivennikov S.I., Tumanov A.V., Drutskaya M.S., Feigenbaum L., Kuprash D.V., Nedospasov S.A. Accelerated thymic atrophy as a result of elevated homeostatic expression of the genes encoded by the TNF/lymphotoxin cytokine locus. Eur J Immunol, 39(10):2906-15, 2009. PMID: 19735075.
- Kruglov, A.A., Kuchmiy, A., Grivennikov, S.I., Tumanov, A.V., Kuprash, D.V., and Nedospasov, S.A. Physiological functions of tumor necrosis factor and the consequences of its pathologic overexpression or blockade: mouse models. Cytokine Growth Factor Rev., 19 (3-4): 231-244, 2008. PMID: 18502680.
- Tumanov A.V., Christiansen P.A., Fu Y-X. The role of lymphotoxin receptor signaling in diseases. Current Mol. Medicine, 7: 567-578, 2007. PMID: 17896993.
- Lo J.C.*, Wang Y.*, Tumanov A.V.*, Bamji M., Yao Z., Reardon C.A., Getz G.S., Fu Y-X. Lymphotoxin beta receptor-dependent control of lipid homeostasis. Science, 316(5822):285-8, 2007. * Contribute equally. PMID: 17431181.
- Zhu M, Chin RK, Tumanov AV, Liu X, Fu YX. Lymphotoxin beta receptor is required for the migration and selection of autoreactive T cells in thymic medulla. J Immunol. 2007 15;179(12):8069-75. PMID: 18056347.
- Lee, Y., Chin, R.K., Christiansen, P., Sun, Y, Tumanov, A.V., Wang, J., Chervonsky, A.V., Fu Y-X. Recruitment and activation of naïve T cells in the islets by lymphotoxin beta receptor dependent tertiary lymphoid structure. Immunity, 25(3): 499-509, 2006. PMID: 16934497.
- Junt, T., Tumanov, A.V., Harris, N., Heikenwalder, M., Zeller N.,, Kuprash D.K., Aguzzi A., Ludewig, B., Nedospasov, S.A., Zinkernagel, R.M. Expression of lymphotoxin beta governs immunity at two distinct levels. Eur J Immunol. 36(8):2061-75, 2006. PMID: 16841297.
- Welniak, L.A., Kuprash D.V., Tumanov, A.V., Panoskaltsis-Mortari A., Blazar, B.R,, Sun K., Nedospasov, S.A, Murphy, W.J. Peyer’s patches are not required for acute graft-versus-host disease after murine allogeneic bone marrow transplantation. Blood. 107(1):410-2, 2006. PMID: 16160014.
- Grivennikov, S.I. *, Tumanov, A.V.*, Liepinsh, D. J., Kruglov, A.A., Marakusha, B.I., Shakhov, A.N., Murakami, T., Drutskaya, L.N., Förster, I., Clausen, B.E., Tessarollo, L., Ryffel, Bernhard, Kuprash, D.V., Nedospasov, S.A. Distinct and non-redundant in vivo functions of TNF produced by T cells and macrophages/neutrophils: protective and deleterious effects. Immunity, 22 (1): 93-104, 2005. *Contribute equally. PMID: 15664162.
- Kuprash, D.V., Tumanov, A.V., Liepinsh, D.J., Koroleva, E.P., Drutskaya, M.S., Kruglov, A.A., Shakhov, A.N., Southon, E., Murphy, W.J., Tessarollo, L., Grivennikov, S.I., Nedospasov, S.A. Novel tumor necrosis factor-knockout mice that lack Peyer’s patches. Eur J Immunol., 35(5): 1592-600, 2005. PMID: 15832287.
- Tumanov, A.V, Kuprash, D.V, Mach, J.A, Nedospasov, S.A, Chervonsky, A.V. Lymphotoxin and TNF produced by B cells are dispensable for maintenance of the follicle-associated epithelium but are required for development of lymphoid follicles in the Peyer’s patches. J Immunol. 173: 86-91, 2004. PMID: 15210762.
- Shakhov, A. N., Rybtsov, S., Tumanov, A. V., Shulenin, S., Dean, M., Kuprash, D. V., and Nedospasov, S. A. SMUCKLER/TIM4 is a distinct member of TIM family expressed by stromal cells of secondary lymphoid tissues and associated with lymphotoxin signaling. Eur J Immunol. 34: 494-503, 2004. PMID: 14768054.
- Tumanov, A.V. Development of secondary lymphoid organs. Review. Immunologya (In russian) 2: 120-131, 2004.
- Tumanov, A. V., Kuprash, D. V., and Nedospasov, S. A. The role of lymphotoxin in development and maintenance of secondary lymphoid tissues. Cytok Growth Factor Rev. 14: 275-288, 2003. PMID: 12787565.
- Tumanov, A. V., Grivennikov, S. I., Shakhov, A. N., Rybtsov, S. A., Koroleva, E. P., Takeda, J., Nedospasov, S. A., and Kuprash, D. V. Dissecting the role of lymphotoxin in lymphoid organs by conditional targeting. Immunol Rev. 195: 106-116, 2003. PMID: 12969314.
- Abe, K., Yarovinsky, F. O., Murakami, T., Shakhov, A. N., Tumanov, A. V., Ito, D., Drutskaya, L. N., Pfeffer, K., Kuprash, D. V., Komschlies, K. L., and Nedospasov, S. A. Distinct contributions of TNF and LT cytokines to the development of dendritic cells in vitro and their recruitment in vivo. Blood. 101: 1477-1483, 2003. PMID: 12560241.
- Tumanov, A. V., Kuprash, D. V., Grivennikov, S. I., Lagarkova, M. A., Abe, K., Shakhov, A. N., Drutskaya, L. N., Stewart, C. L., Chervonsky, A. V., and Nedospasov, S. A. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity. 17: 239-250, 2002. PMID: 12354378.
- Kuprash, D. V. *, Alimzhanov, M. B. *, Tumanov, A. V. *, Shakhov, A. N., Grivennikov, S. I., Marino, M. W., Turetskaya, R. L., Anderson, A. O., Rajewsky, K., Pfeffer, K., and Nedospasov, S. A. Redundancy in TNF and LT signaling in vivo: mice with inactivation of the entire TNF/LT locus versus single knockout mice. Cell. Biol. 22: 8626-8634, 2002. *Contribute equally. PMID: 12446781.
- Le Y, Gong W, Tiffany H.L., Tumanov A.V, Nedospasov S.A, Shen W., Dunlop N.M, Gao J.L., Murphy P.M., Oppenheim J.J., Wang J.M. Amyloid (beta)42 activates a G-protein-coupled chemoattractant receptor, FPR-like-1. J Neurosci. 21(2): RC123, 2001. PMID: 11160457.
- Shakhov, A. N., Lyakhov, I. G., Tumanov, A. V., Rubtsov, A. V., Drutskaya, L. N., Marino, M. J., and Nedospasov, S. A. Gene profiling approach in the analysis of lymphotoxin and TNF deficiencies. Leuk. Biol. 68: 151-157, 2000. PMID: 10914503.
- Kuprash, D. V. *, Alimzhanov, M. B. *, Tumanov, A. V. *, Rajewsky, K., Anderson, A. O., Pfeffer, K., and Nedospasov, S. A. TNF and lymphotoxin beta cooperate in the maintenance of secondary lymphoid tissue microarchitecture but not in the development of lymph nodes. Immunol. 163(12):6575-80 163: 6575-6580, 1999. *Contribute equally. PMID: 10586051.
- Shakhov, A.N., Rubtsov, A.V, Lyakhov, I.G., Tumanov, A.V., Nedospasov S.A. SPLASH (PLA2IID), a novel member of phospholipase A2 family, is associated with lymphotoxin deficiency. Genes Immun, 1(3): 191-9, 2000. PMID: 11196711.
- Tumanov, A.V., Nedospasov, S.A., Turetskaya, R.L. Chromatin organization in the tumor necrosis factor/lymphotoxin gene locus: correlation with tissue-specific expression. Molecular Biology (in Russian), 32(1): 102-106, 1998. PMID: 9566256.
- Kovalchuk, L.V., Khoroshilova-Maslova, I.P., Gankovskaya, L.V., Krainova, T.A., Gundorova, R.A., Ilatovskaya L.V., Marteushev A.V., Tumanov, A.V. Natural complex of cytokines is a potent stimulant to posttraumatic regeneration in rabbit cornea. J Ocul Pharmacol Ther.,12(3):271-9, 1996. PMID: 8875333.

