
Contact
- (210) 567-3923
- leadbetter@uthscsa.edu
Department
Microbiology, Immunology & Molecular GeneticsLeadbetter, Elizabeth A., Ph.D.
Associate Professor
Personal Statement:
The Leadbetter laboratory is pursuing the following two long term research goals, to thoroughly investigate the mechanisms and nature of cooperation between iNKT and B cells during chronic inflammation and to extend these findings to inform the development of a new family of lipid-based immunotherapy candidates for protection against infection and tumors.
I am actively studying B/iNKT cooperation using murine models of vaccination, infection, autoimmunity, and metabolic disease, including translational applications into glycolipid-loaded nanoparticle-based tumor immunotherapy. We specialize in spectral flow cytometry, in vivo cellular immunology, and in situ imaging. Our work takes advantage of the abundant intellectual and material resources in the Lozano Long School of Medicine including colleagues in the Department of Microbiology, Immunology & Molecular Genetics, but also the Diabetes Division, the Texas Diabetes Institute, the UT Health San Antonio MD Anderson Mays Cancer Center as well as the Cancer Therapy & Research Center (CTRC). Our research is further enriched by the many clinical practices of the associated University Hospital which manage one of the largest populations of obese/Type 2 diabetes patients in the US, with associated specialization in related cancers and metabolic diseases. This combination of competitive basic research, translational animal models, and extensive clinical experience places San Antonio at the nexus of translational research into obesity and associated comorbidities.
Education
PhD, Microbiology and Immunology at Boston University School of Medicine, Boston, MA
Postdoctoral Fellowship, Brigham & Women’s Hospital/Harvard Medical School, Boston, MA
Research
 The Leadbetter laboratory has two long term research goals: to investigate the mechanisms and nature of cooperation between iNKT and B cells during acute responses (infection) and chronic inflammation (autoimmunity and obesity) and to translate this knowledge into a new family of lipid-based vaccine candidates and/or autoimmune interventions.
The Leadbetter laboratory has two long term research goals: to investigate the mechanisms and nature of cooperation between iNKT and B cells during acute responses (infection) and chronic inflammation (autoimmunity and obesity) and to translate this knowledge into a new family of lipid-based vaccine candidates and/or autoimmune interventions. 
Leukocyte interplay during the chronic inflammation of obesity
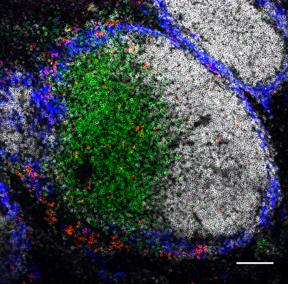
 Obesity is linked to chronic, low-grade inflammation in visceral adipose tissue and an associated spectrum of metabolic abnormalities including insulin resistance and T2D. We previously discovered that neutrophils license iNKT cells to regulate autoreactive B cells during IL-18-mediated chronic inflammation in the spleen, but crosstalk between these cell subsets remains unexplored in the setting of obesity and T2D. The Leadbetter lab was the first in the world to develop an innovative confocal imaging technique to identify iNKT cells in situ using fresh tissue sections. We now integrate this approach with sophisticated in vivo murine models including high fat diet-induced obesity, cytokine reporter mouse strains, and mixed bone marrow chimeras to study adipose leukocytes. We complement our murine studies with 13+ color flow cytometry of ex vivo and in vitro cultured human lymphocytes isolated from the adipose tissue of lean and obese patients. This project is dissecting the interplay of iNKT cells, neutrophils, and B cells in the context of both murine and human obesity to understand the immunological implications of this public health epidemic and contribute to high priority national and international efforts to stem the tide of obesity. Our objective is to define new immunotherapeutic targets to reverse the development of T2D in obesity. Our location in San Antonio has uniquely positioned us to succeed in performing these studies. San Antonio was recently identified as having the second highest level of obesity of any major city in the U.S. (>31%) so clinical samples and clinical practicioners with relevant obesity and T2D expertise are readily accessible. Dr. Michael Decherd (San Antonio Plastic Surgery Institute), Dr. Robert Schenken (Obstetrics and Gynecology; UTHSCSA), and Dr. Richard Peterson (Bariatric and Metabolic Surgery UT Medicine) provide subcutaneous and omental adipose tissue from patients undergoing elective surgeries in their practices. We routinely analyze fresh human adipose samples from both lean and obese patients. Hagglof, T; Sedimbi, SK; Yates, JL; Parsa, R; Salas, BH; Harris, RA; Leadbetter, EA*; Karlsson, MCI*. Neutrophils license iNKT cells to regulate self-reactive mouse B cell responses. Nature Immunology 2016; advanced online publication Oct. 31, 2016. *contributed equally. Lynch L; Michelet X; Zhang S; Brennan PJ; Moseman A; Lester C; Vomhof-Dekrey EE; Tighe M; Koay HF; Godfrey DI; Leadbetter EA; Sant’Angelo DB; von Andrian U; Brenner MB. Regulatory iNKT cells lack PLZF and control Treg and macrophage homeostasis in adipose tissue. Nature Immunology 2015 Jan;16(1):85-95. doi: 10.1038/ni.3047. Epub 2014 Dec 1. King IL, Amiel E, Tighe M, Mohrs K, Veerapen N, Besra G, Mohrs M, Leadbetter EA. The mechanism of splenic invariant NKT cell activation dictates localization in vivo. J Immunol. 2013 Jul 15;191(2):572-82. Epub 2013 Jun 19.
Obesity is linked to chronic, low-grade inflammation in visceral adipose tissue and an associated spectrum of metabolic abnormalities including insulin resistance and T2D. We previously discovered that neutrophils license iNKT cells to regulate autoreactive B cells during IL-18-mediated chronic inflammation in the spleen, but crosstalk between these cell subsets remains unexplored in the setting of obesity and T2D. The Leadbetter lab was the first in the world to develop an innovative confocal imaging technique to identify iNKT cells in situ using fresh tissue sections. We now integrate this approach with sophisticated in vivo murine models including high fat diet-induced obesity, cytokine reporter mouse strains, and mixed bone marrow chimeras to study adipose leukocytes. We complement our murine studies with 13+ color flow cytometry of ex vivo and in vitro cultured human lymphocytes isolated from the adipose tissue of lean and obese patients. This project is dissecting the interplay of iNKT cells, neutrophils, and B cells in the context of both murine and human obesity to understand the immunological implications of this public health epidemic and contribute to high priority national and international efforts to stem the tide of obesity. Our objective is to define new immunotherapeutic targets to reverse the development of T2D in obesity. Our location in San Antonio has uniquely positioned us to succeed in performing these studies. San Antonio was recently identified as having the second highest level of obesity of any major city in the U.S. (>31%) so clinical samples and clinical practicioners with relevant obesity and T2D expertise are readily accessible. Dr. Michael Decherd (San Antonio Plastic Surgery Institute), Dr. Robert Schenken (Obstetrics and Gynecology; UTHSCSA), and Dr. Richard Peterson (Bariatric and Metabolic Surgery UT Medicine) provide subcutaneous and omental adipose tissue from patients undergoing elective surgeries in their practices. We routinely analyze fresh human adipose samples from both lean and obese patients. Hagglof, T; Sedimbi, SK; Yates, JL; Parsa, R; Salas, BH; Harris, RA; Leadbetter, EA*; Karlsson, MCI*. Neutrophils license iNKT cells to regulate self-reactive mouse B cell responses. Nature Immunology 2016; advanced online publication Oct. 31, 2016. *contributed equally. Lynch L; Michelet X; Zhang S; Brennan PJ; Moseman A; Lester C; Vomhof-Dekrey EE; Tighe M; Koay HF; Godfrey DI; Leadbetter EA; Sant’Angelo DB; von Andrian U; Brenner MB. Regulatory iNKT cells lack PLZF and control Treg and macrophage homeostasis in adipose tissue. Nature Immunology 2015 Jan;16(1):85-95. doi: 10.1038/ni.3047. Epub 2014 Dec 1. King IL, Amiel E, Tighe M, Mohrs K, Veerapen N, Besra G, Mohrs M, Leadbetter EA. The mechanism of splenic invariant NKT cell activation dictates localization in vivo. J Immunol. 2013 Jul 15;191(2):572-82. Epub 2013 Jun 19.
Cooperation of NKT cells and B cells in response to lipid antigens
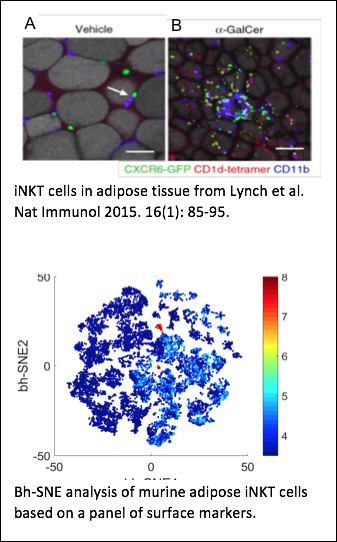
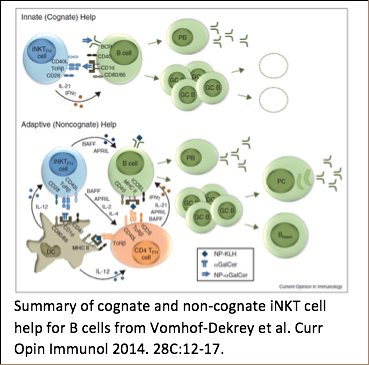 Different from protein-reactive T cells, iNKT cells are a unique cell population that recognizes lipids in the context of the CD1 family of antigen presenting molecules. Certain B cells recognize and internalize lipids and also express CD1 molecules on their surface, which makes them ideal partners for iNKT cells. Upon activation, iNKT cells express co-stimulatory molecules and release cytokines which helps CD1d-expressing B cells to proliferate and increase their secretion of antibodies. Thus, iNKT cells can improve a B cell’s response to antigens that they recognize in common. Dr. Leadbetter’s lab has already demonstrated that this cooperation happens using a model lipid conjugate. However, B cells receiving cognate iNKT cell help have a profoundly different developmental outcome than B cells receiving non-cognate help involving both iNKT cells and protein-reactive CD4+ T cells. Cognate iNKT cell help drives a rapid, but un-sustained, GC B cell expansion but no B cell memory. Non-cognate iNKT cell help drives later GC B cell expansion more consistent with protein responses and induces a robust B cell memory response. We are using complex in vivo murine models, unique haptenated glycolipids, flow cytometry, and RNA array analysis to investigate the mechanisms of iNKT cell help which mediate this difference. Long term goals are to understand the interplay of iNKT and B cells and to harness this unique system to understand the global requirements for humoral B cell memory. Vomhof-Dekrey EE; Yates J; Hägglöf T; Lanthier P; Amiel E; Veerapen N; Besra GS; Karlsson MCI; Leadbetter EA. Cognate interaction with iNKT cells activates B cells to produce IL-10. Proc Natl Acad Sci USA. 2015 Oct 6;112(40):12474-9. doi: 10.1073/pnas.1504790112. Epub 2015 Sep 21. Vomhof-DeKrey EE; Yates J; Leadbetter EA. Invariant NKT cells provide innate and adaptive help for B cells. Curr Opin Immunol. 2014 Feb 7;28C:12-17. doi: 10.1016/j.coi.2014.01.007. King IL, Fortier A, Tighe M, Dibble J, Watts G, Veerapen N, Haberman A, Besra GS, Mohrs M, Brenner MB, Leadbetter, EA. iNKT cells direct B cell responses to cognate lipid antigen in an interleukin 21-dependent manner. Nature Immunology 2011; Nov 27, 13(1): 44-50. *Nature Immunology News & Views 2012; 13(1): 11-13. *Nature Reviews Immunology 2012; 12(1):5. Leadbetter EA, Brigl M, Illarionov P, Cohen N, Luteran M, Pillai S, Besra G, Brenner MB. NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci USA 2008; Jun 17, 105(24):8339-44. Epub 2008 Jun 11.
Different from protein-reactive T cells, iNKT cells are a unique cell population that recognizes lipids in the context of the CD1 family of antigen presenting molecules. Certain B cells recognize and internalize lipids and also express CD1 molecules on their surface, which makes them ideal partners for iNKT cells. Upon activation, iNKT cells express co-stimulatory molecules and release cytokines which helps CD1d-expressing B cells to proliferate and increase their secretion of antibodies. Thus, iNKT cells can improve a B cell’s response to antigens that they recognize in common. Dr. Leadbetter’s lab has already demonstrated that this cooperation happens using a model lipid conjugate. However, B cells receiving cognate iNKT cell help have a profoundly different developmental outcome than B cells receiving non-cognate help involving both iNKT cells and protein-reactive CD4+ T cells. Cognate iNKT cell help drives a rapid, but un-sustained, GC B cell expansion but no B cell memory. Non-cognate iNKT cell help drives later GC B cell expansion more consistent with protein responses and induces a robust B cell memory response. We are using complex in vivo murine models, unique haptenated glycolipids, flow cytometry, and RNA array analysis to investigate the mechanisms of iNKT cell help which mediate this difference. Long term goals are to understand the interplay of iNKT and B cells and to harness this unique system to understand the global requirements for humoral B cell memory. Vomhof-Dekrey EE; Yates J; Hägglöf T; Lanthier P; Amiel E; Veerapen N; Besra GS; Karlsson MCI; Leadbetter EA. Cognate interaction with iNKT cells activates B cells to produce IL-10. Proc Natl Acad Sci USA. 2015 Oct 6;112(40):12474-9. doi: 10.1073/pnas.1504790112. Epub 2015 Sep 21. Vomhof-DeKrey EE; Yates J; Leadbetter EA. Invariant NKT cells provide innate and adaptive help for B cells. Curr Opin Immunol. 2014 Feb 7;28C:12-17. doi: 10.1016/j.coi.2014.01.007. King IL, Fortier A, Tighe M, Dibble J, Watts G, Veerapen N, Haberman A, Besra GS, Mohrs M, Brenner MB, Leadbetter, EA. iNKT cells direct B cell responses to cognate lipid antigen in an interleukin 21-dependent manner. Nature Immunology 2011; Nov 27, 13(1): 44-50. *Nature Immunology News & Views 2012; 13(1): 11-13. *Nature Reviews Immunology 2012; 12(1):5. Leadbetter EA, Brigl M, Illarionov P, Cohen N, Luteran M, Pillai S, Besra G, Brenner MB. NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci USA 2008; Jun 17, 105(24):8339-44. Epub 2008 Jun 11.
Glycolipid-based vaccine strategies for tumor immunotherapy
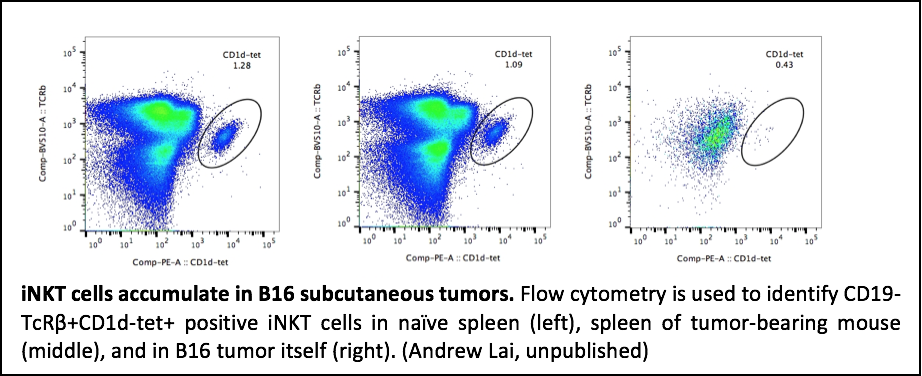 Current cancer immunotherapy strategies employ monoclonal antibodies, antitumor vaccines, and vaccine adjuvants in order to stimulate a patient’s own immune system to combat cancer. However, there is a critical need for additional therapeutic approaches as an effective, targeted immune response is crucial in order to eliminate a well-established, proliferating tumor. Invariant natural killer (iNK) T cells, a subset of T cells, are a potential immunotherapeutic target as they bridge cellular and humoral immunity by both directly killing tumor cells and facilitating protective immune responses of CD8+ T through direct and indirect killing. The most well studied glycolipid, alpha-galactosylceramide (αGalCer), is capable of rapidly activating iNKT cells to proliferate and secrete Th1 and Th2-type cytokines. αGalCer has also been utilized as a vaccine adjuvant to induce both humoral and cellular antitumor immune responses; however, high doses of αGalCer provide vigorous initial activation of iNKT cells, but subsequent injections result in anergy, or inactivation. To overcome this limitation, our lab has recently used biodegradable nanoparticles to deliver low doses of glycolipid adjuvant in combination with pathogen-derived antigens to successfully protect against lethal infection without inducing iNKT anergy. We hypothesize that this approach will be equally effective in driving responses to tumor antigens. Nanoparticles are safe, biodegradable polymers able to enhance the activity of encapsulated glycolipid adjuvants 1000-fold. Furthermore, nanoparticles can be embedded with pathogen-derived antigens to facilitate activation of relevant CD8+ T cell subpopulations. We are using nanoparticle delivery of low doses of glycolipid adjuvant in combination with tumor associated antigens (TAA) to circumvent iNKT anergy and induce protective IgG and CD8+ T cell responses against established tumors.
Current cancer immunotherapy strategies employ monoclonal antibodies, antitumor vaccines, and vaccine adjuvants in order to stimulate a patient’s own immune system to combat cancer. However, there is a critical need for additional therapeutic approaches as an effective, targeted immune response is crucial in order to eliminate a well-established, proliferating tumor. Invariant natural killer (iNK) T cells, a subset of T cells, are a potential immunotherapeutic target as they bridge cellular and humoral immunity by both directly killing tumor cells and facilitating protective immune responses of CD8+ T through direct and indirect killing. The most well studied glycolipid, alpha-galactosylceramide (αGalCer), is capable of rapidly activating iNKT cells to proliferate and secrete Th1 and Th2-type cytokines. αGalCer has also been utilized as a vaccine adjuvant to induce both humoral and cellular antitumor immune responses; however, high doses of αGalCer provide vigorous initial activation of iNKT cells, but subsequent injections result in anergy, or inactivation. To overcome this limitation, our lab has recently used biodegradable nanoparticles to deliver low doses of glycolipid adjuvant in combination with pathogen-derived antigens to successfully protect against lethal infection without inducing iNKT anergy. We hypothesize that this approach will be equally effective in driving responses to tumor antigens. Nanoparticles are safe, biodegradable polymers able to enhance the activity of encapsulated glycolipid adjuvants 1000-fold. Furthermore, nanoparticles can be embedded with pathogen-derived antigens to facilitate activation of relevant CD8+ T cell subpopulations. We are using nanoparticle delivery of low doses of glycolipid adjuvant in combination with tumor associated antigens (TAA) to circumvent iNKT anergy and induce protective IgG and CD8+ T cell responses against established tumors.
Glycolipid-based vaccine strategies for infectious disease protection
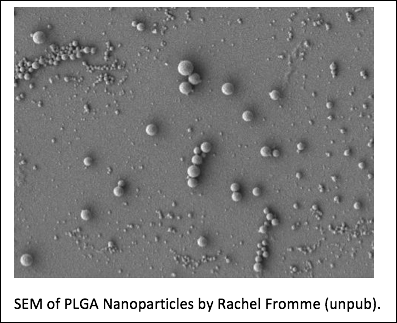 Most successful human vaccines elicit protective antibodies. CD4+ T cell help is essential for a strong humoral immune response, especially against poorly immunogenic polysaccharide antigens. To accomplish this, B cell antigens are typically conjugated to highly immunogenic T cell epitopes. iNKT cells are an innate glycolipid-specific lymphocyte subset of T cells restricted to the non-polymorphic antigen presenting molecule CD1d, which rapidly produce cytokines when activated. Because CD1d is non-polymorphic iNKT cells should respond to glycolipid stimulation equally well in everyone. Relatedly, we and others have recently shown that by activating iNKT cells, glycolipids can serve as robust adjuvants for co-administered protein and polysaccharide antigens. However, high doses of glycolipid tend to induce an anergic state in iNKT cells. To overcome this limitation, low doses of iNKT-activating glycolipids need to be administered in a highly efficient manner. Nanoparticle vaccines are a well-studied platform for delivering low doses of glycolipid adjuvant. Nanoparticles are safe, biodegradable polymers able to enhance the activity of encapsulated glycolipid adjuvants 1000-fold. Furthermore, nanoparticles can be embedded with B cell antigens to facilitate their targeting and uptake by relevant B cell subpopulations. We are currently testing nanoparticle delivery of low doses of glycolipid adjuvant with polysaccharide B cell antigen can circumvent anergy and induce protective IgG against systemic infection with an encapsulated pathogen, Streptococcus pnemoniae. This is a proof of principle study which will test a universal approach that could apply to most types of bacteria, fungi, parasites, or other pathogens.
Most successful human vaccines elicit protective antibodies. CD4+ T cell help is essential for a strong humoral immune response, especially against poorly immunogenic polysaccharide antigens. To accomplish this, B cell antigens are typically conjugated to highly immunogenic T cell epitopes. iNKT cells are an innate glycolipid-specific lymphocyte subset of T cells restricted to the non-polymorphic antigen presenting molecule CD1d, which rapidly produce cytokines when activated. Because CD1d is non-polymorphic iNKT cells should respond to glycolipid stimulation equally well in everyone. Relatedly, we and others have recently shown that by activating iNKT cells, glycolipids can serve as robust adjuvants for co-administered protein and polysaccharide antigens. However, high doses of glycolipid tend to induce an anergic state in iNKT cells. To overcome this limitation, low doses of iNKT-activating glycolipids need to be administered in a highly efficient manner. Nanoparticle vaccines are a well-studied platform for delivering low doses of glycolipid adjuvant. Nanoparticles are safe, biodegradable polymers able to enhance the activity of encapsulated glycolipid adjuvants 1000-fold. Furthermore, nanoparticles can be embedded with B cell antigens to facilitate their targeting and uptake by relevant B cell subpopulations. We are currently testing nanoparticle delivery of low doses of glycolipid adjuvant with polysaccharide B cell antigen can circumvent anergy and induce protective IgG against systemic infection with an encapsulated pathogen, Streptococcus pnemoniae. This is a proof of principle study which will test a universal approach that could apply to most types of bacteria, fungi, parasites, or other pathogens.
Group Members
Elizabeth Leadbetter  Elizabeth Leadbetter graduated from Bates College in Lewiston, Maine in 1993, worked at Immulogic Pharmaceutical Corp, and then received her Ph.D. in 2002 from Boston University School of Medicine where she studied with Dr. Ann Marshak-Rothstein. She conducted her postdoctoral research at Brigham & Women’s Hospital; Harvard Medical School, Boston, MA in the lab of Dr. Michael Brenner. In 2009, she joined the faculty at the Trudeau Institute in Saranac Lake, NY as an Assistant Member. In 2015 she moved her laboratory to the UT Health San Antonio where she is currently an Associate Professor. She is a member of the American Association of Immunologists and currently serves as chair of the MIMG department Faculty Recruitment Committee.
Elizabeth Leadbetter graduated from Bates College in Lewiston, Maine in 1993, worked at Immulogic Pharmaceutical Corp, and then received her Ph.D. in 2002 from Boston University School of Medicine where she studied with Dr. Ann Marshak-Rothstein. She conducted her postdoctoral research at Brigham & Women’s Hospital; Harvard Medical School, Boston, MA in the lab of Dr. Michael Brenner. In 2009, she joined the faculty at the Trudeau Institute in Saranac Lake, NY as an Assistant Member. In 2015 she moved her laboratory to the UT Health San Antonio where she is currently an Associate Professor. She is a member of the American Association of Immunologists and currently serves as chair of the MIMG department Faculty Recruitment Committee.
Awards & Accomplishments
- AAI Careers in Immunology Fellowship (w/Raksha Parthasarathy) 2021-2022, American Association of Immunologists, Bethesda, Maryland
- AAI Early Career Faculty Travel Award 2014, American Association of Immunologists, Bethesda, Maryland
- AAI Public Policy Fellowship 2013, American Association of Immunologists, Bethesda, Maryland
- Keystone Symposia Scholarship, Keystone Symposia, Silverthorne, CO 2006
- Postdoctoral Fellowship (Biogen/IDEC Foundation Fellow), Irvington Institute for Immunologic Research, NY, NY 2004-2007
- President’s Award, Boston University Science and Technology Day, 2002
- Keystone Symposia Student Travel Award, Keystone Symposia, Silverthorne, CO 2002
- Microbiology Department Graduate Student Travel Award, Boston University School of Medicine, 2002
- Lawrence M. Corwin Graduate Student Award, Boston University School of Medicine, Department of Microbiology, 2001
- Henry I. Russek Student Achievement Award 2nd place, Boston University School of Medicine, 2000, 2001
Affiliations
- Member, American Association of Immunologist (AAI)
Associated Core Facilities (http://iims.uthscsa.edu/ttr_core_facilities.html)
- UTHSCSA Flow Cytometry Core: (http://research.uthscsa.edu/facs/)
- UTHSCSA Optical Imaging Facility: (http://uthscsa.edu/csa/imaging.asp)
- Barshop Institute for Longevity and Aging Studies: (http://barshopinstitute.uthscsa.edu/main/core)
- UTHSCSA Bioanalytics and Single-Cell Core: (http://molecularmedicine.uthscsa.edu/research.aspx)
- UTHSCSA Mass Spectrometry/Lipidomics Core: (http://research.uthscsa.edu/mass-spectrometry/)
- UTHSCSA Genomics Resources Core: (http://uthscsa.edu/csa/genomics.asp)
Lab Members
Current Lab Members
| Picture | Contact |
|---|---|
| Liz Dudley, MS Research Technician dudleye@uthscsa.edu |
|
 | Ben Enslow, MD Postdoctoral Fellow enslow@uthscsa.edu |
 | Carlo Vanz, MBA Graduate Student vanz@livemail.uthscsa.edu |
| Nathaniel Liendo MARC Undergraduate Student, St.Mary's College |
Leadbetter Lab Alumni
| Name | Current Position |
|---|---|
| Noran Alam | MS |
| Eyal Amiel | PhD Associate Professor; University of Vermont, Burlington, VT |
| Anne Fortier | PhD Research Scientist; Vertex Pharmaceuticals Corp., Montreal, CN |
| Anne Fortier | PhD Research Scientist; Vertex Pharmaceuticals Corp., Montreal, CN |
| Thomas Hägglöf | PhD Postdoctoral Fellow, Rockefeller University, NY, NY |
| Briana Hauf Salas | PhD Assistant Professor, Our Lady of the lake University, San Antonio, TX |
| Abigail Koenigs | Undergraduate Researcher, Univ of Incarnate Word, San Antonio, TX |
| Abigail Kumagai | Wayne State University Medical School Student, Detroit, MI |
| Andrew Lai | MS |
| Chloe Mesa | MS |
| Vanessa Ortega | MS |
| Raksha Parthasarathy | PhD Scientist, 23 and Me; San Francisco, CA |
| Adri Serrata | MS |
| Travis Shute | PhD Scientist, Tessera Therapeutics, Boston, MA |
| Emilie Vomhof-Dekrey | PhD Research Assistant Professor; University of North Dakota, Grand Forks, ND |
| Jennifer Yates | PhD Research Scientist; Wadsworth Center, Albany, NY |
Publications
Current Active Grants:
R01 AI132798-01 (Leadbetter, PI) 12/2017-11/2022. NIAID/NIH
Innate modulation of autoimmune, regulatory, and effector B cells in adipose tissue; This project will identify key interactions between leukocytes which mediate inflammation in adipose tissue during obesity.
Up-to-date listing of Leadbetter publications on PubMed: http://www.ncbi.nlm.nih.gov/sites/myncbi/elizabeth.leadbetter.1/bibliograpahy/40852781/public/?sort=date&direction=ascending
Recent Publications:
Hägglöf, T.; Vanz, C.; Kumagai, A.; Keegan, J.; Koenigs, A.; Leadbetter, EA. iNKT-cell dependent Tbet+ B cells accumulate in adipose tissue and exacerbate metabolic disease. Cell Metabolism; 2022 Aug 2, 34 (8): 1121-1136. https://doi.org/10.1016/j.cmet.2022.07.002.
Parthasarathy, R.; Hägglöf, T.; Hadley, J.; McLennan, A.; Mattke, A.; Dudley, E.; Kumagai, A.; Dong, LQ; Leadbetter, EA. Receptor interacting protein kinase pathways regulate innate B cell developmental checkpoints but not effector function. Frontiers in Immunology, 9 Dec 2021. https://doi.org/10.3389/fimmu.2021.758407
Shou, Y.; Koroleva, E.; Spencer, CM.; Shein, SA.; Korchagina, AA.; Yusoof, KA.; Parthasarathy, R.; Leadbetter, EA.; Akopian, AN.; Munoz, AP.; Tumanov, AV. Redefining the role of Lymphotoxin Beta Receptor in the maintenance of lymphoid organs and immune cell homeostasis in adulthood. Front Immunol. 2021; Frontiers in Immunology 2021 Jul 15;12:712632. doi: 10.3389/fimmu.2021.712632. eCollection 2021.PMID: 34335629
Lee, G.C. et al. Immunologic Resilience and COVID-19 survival advantage. Journal of Allergy and Clinical Immunology, 148 (5), 2021; pp. 1176-1191.
Shute T.; Amiel, E.; Alam, N.; Yates, JL.; Mohrs, K.; Dudley E.; Salas, B.; Mesa, C.; Serrata, A.; Angel, D.; Vincent, BK.; Weyers, A.; Lanthier, PA.; Vomhof-Dekrey, E.; Fromme, R.; Laughlin, M.; Durham, O.; Miao, J.; Shipp, D.; Linhardt, RJ.; Nash, K.; Leadbetter, EA. Glycolipid-containing nanoparticle vaccine engages iNKT cells to enhance humoral protection against systemic bacterial infection but abrogates T-independent vaccine responses. Journal of Immunology; 2021; April, 206: 1-11. DOI: https://doi.org/10.4049/jimmunol.2001283
Leadbetter, EA and Karlsson MCI. Reading the room: iNKT cells influence B cell responses. Molecular Immunology. 2021; Feb, 130: 49-54. Epub 2020 Dec 22. DOI: 10.1016/j.molimm.2020.12.005
Leadbetter, EA and Karlsson MCI. Invariant natural killer T cells balance B cell immunity. Immunological Reviews 2021; Jan, 299(1): 93-107. doi.org/10.1111/imr.12938
Sedimbi, S.; Hägglöf, T.; Garimella, M.; Wang, S.; Duhlin, A.; Coelho, A.; Ingelshed, K.; Mondoc, E.; Malin, S.G.; Holmdahl, R.; Lane, D.P.; Leadbetter, EA.; Karlsson, MCI. Combined proinflammatory cytokine and cognate activation of invariant natural killer T cells enhances anti-DNA responses. PNAS April 15, 2020.
Lin, J.; Mohrs, K.; Szaba, F.; Kummer, L.; Leadbetter, EA.; Mohrs, M. Virtual memory CD8 T cells expanded by helminth infection confer broad protection against bacterial infection. Mucosal Immunology 2019; Jan, 12(1): 258-264. Epub 2018 Oct 25. Doi: 10.1038/s41385-018-0100-x.
Yates, JL.; Leadbetter, E.A.; Mantis, N.J. Alpha-galactosylceramide (aGalCer) enhances vaccine induced protection in a model of ricin intoxication. Hum Vaccin Immunother 2018; 14(8): 2053-2057.
King IL; Meli AP; Downey J; Lanthier P; Tzelepis F; Fritz JH; Tumanov AV; Divangahi M; Leadbetter EA; Mohrs M. Intestinal helminth infection impacts the systemic distribution and function of the naïve lymphocyte pool. Mucosal Immunology 2017; Advance Online Publication Jan 25: doi: 10.1038/mi.2016.127.
Hagglof, T; Sedimbi, SK; Yates, JL; Parsa, R; Salas, BH; Harris, RA; Leadbetter, EA*; Karlsson, MCI*. Neutrophils license iNKT cells to regulate self-reactive mouse B cell responses. Nature Immunology 2016; advanced online publication Oct. 31, 2016. *contributed equally.
