(Aliases: 18q deletion syndrome, partial monosomy 18q, de Grouchy Syndrome, chromosome 18 long-arm deletion syndrome)
ICD-10 = Q99.9 or Q93.89
Key points on genotype
- Every patient has a unique deletion; thus 18q- is not a single “syndrome.” (For guidance developing an individualized gene-based interpretation see subsequent pages.)
- Some genotype-phenotype correlations have been established and are explored more in the following pages.
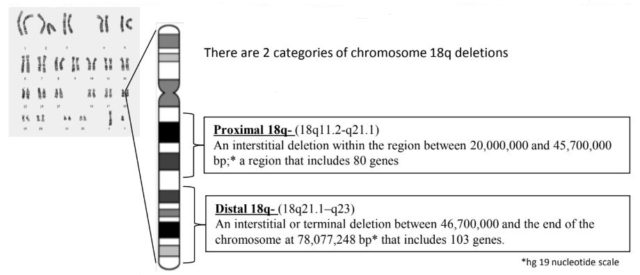
- ~19% are interstitial deletions
- ~8% with terminal deletions have duplications just proximal to the breakpoint of up to 15 Mb in size
- 94% are de novo events as opposed to inherited from a parent with a translocation.
- Occurs in 1 out of 55,000 live births
Key Points on phenotype
- Multiple congenital anomalies are possible. Specific phenotypes are dependent on the specific genes deleted – see the section of this report on molecular implications.
- Developmental milestones are always delayed but with considerable variability
- Intellectual disability is common but not inevitable
- Failure to thrive and growth hormone deficiency are common
- The risk for autism spectrum is higher than average
- Life expectancy is believed to be near normal except for individuals whose deletion includes TCF4.
- Deletions that include the TCF4 gene (at band q21.2; or at 52.9 Mb) result in a much more severe phenotype, making it important to determine if someone’s deletion includes this gene.
Management
- Affected individuals are not at increased risk for adverse reactions to drugs or standard medical treatments
- Treatment is primarily symptomatic
- Recommendations for specific evaluations and treatments are in the following sections
Enrollment
- The Chromosome 18 Clinical Research Center is enrolling anyone with any chromosome 18 abnormality in our longitudinal study of all aspects of the conditions.
- Parents need to contact Annice Hill at hilla3@uthscsa.edu or call (210) 567-5321
- We need the diagnostic genetics report and any other informative medical records
- Then we will schedule a blood draw and shipment
Consultation
- Daniel Hale, MD, Medical Director of the Chromosome 18 Clinical Research Center can be reached through Annice Hill at hilla3@uthscsa.edu or call (210) 567-5321.
Updated 2020