Technology
Overview
Cryo-EM Facility
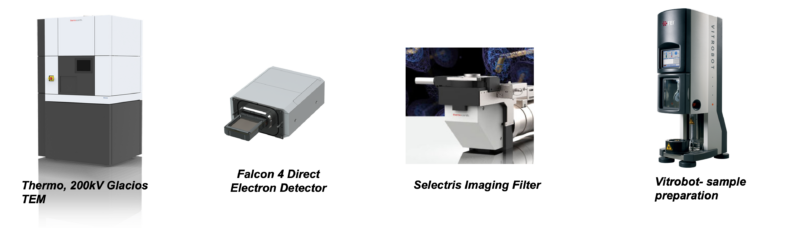
The Cryo-EM facility is a state-of-the-art laboratory equipped with a Glacios TEM, Falcon 4 detector, and Selectris energy filter – the first setup of its kind in the region. The facility is fully capable of supporting all steps of structure determination using single particle cryo-EM. It includes a Vitrobot and Leica Plunge Freezer for grid preparation.
SBC High-Performance Computing Cluster
Our 36-GPU-equipped high-performance computing cluster enables investigators to leverage state-of-the art software for all three major branches of structural biology including cryo-EM, X-ray crystallography, and NMR as well as artificial intelligence-based approaches to in silico modeling of multiprotein complex structures. The cluster includes high-throughput 1.5PB storage with commonly used cryo-EM software pre-installed. The HPC was funded by NIH-ORIP Shared Instrumentation Grant S10OD036251.
X-ray Crystallography Facility
The X-ray Crystallography Facility is fully equipped to carry out all aspects of protein crystallography. The equipment infrastructure presently includes a state-of- the-art Rigaku HyPix 6000HE hybrid photon counting detector, VariMax-VHF confocal microfocus optic and Universal Kappa Goniometer (funded by NIH-ORIP Shared Instrumentation Grant S10OD030374) mounted on our high-flux Rigaku MicroMax 007-HF X-ray generator. Crystals are cooled during data acquisition with an Oxford Cryostream 800 cryogenic system. A Linux computing cluster is also in place enabling the complete protein structure determination and refinement process to be accomplished in relatively short order.
We use an Art Robbins Instruments Phoenix pipetting robot for rapid crystallization screening using 96-well sitting drop plates with the ability to accurately and reproducibly dispense <200 nanoliter volumes. An Art Robbins Scorpion Screen Builder is available for developing crystal optimization screens and for single-channel liquid handling. In addition, the facility uses a Formulatrix Rock Imager 54 which has the ability to automatically take high resolution, composite photographs of each crystallization experiment in a 96-well plate.

Hybrid Photon Counting Detector

High-throughput Crystallization

Automated Crystal Imaging
NMR Facility
The Biomolecular NMR facility includes two state-of-the-art Bruker Avance NMR spectrometers – a Bruker Avance III 500 MHz and a Bruker Avance Neo 700 MHz. The spectrometers are each equipped with four independent RF channels, triple-axis pulsed field gradients, deuterium decoupling capability, a variable temperature controller, and high sensitivity cryogenically cooled 1H/13C/15N probes (1.7 mm ‘TCI’ on the 500 and a 5.0 mm ‘TCI’ on the 700). The 500 MHz NMR is fitted with an automated sample changer than can handle up to 500 1.7-mm tubes. As configured, the 500 is ideal for assigning smaller proteins (less than 25 kDa) and natural products, and for carrying out NMR-based fragment screening; the 700 is ideally configured for assigning larger macromolecules and related complexes with therapeutic agents, including small molecules, purified natural products, and peptides. The facility also houses a Jasco 815 Circular Dichroism spectrometer which is suitable for structure analysis and thermal stability of proteins and nucleic acids.


Protein Production Facility
The protein production facility is equipped for overexpressing protein targets in E. coli, insect cells and mammalian cells. Core personnel will assist users in plasmid construct design and will run test expression and purifcation schemes to generate soluble protein samples. Soluble proteins may be optimized to generate samples suitable for structural biology and high-throughput screening applications. Protein refolding may also be explored where applicable.
Contact
Shaun Olsen, Ph.D.
Director
210-450-3091
Email: OlsenS@uthscsa.edu
Elizabeth Wasmuth, Ph.D.
Cryo-EM Facility Co-Director
210-567-5965
Email: WasmuthE@uthscsa.edu
Alex Taylor, Ph.D.
Associate Director
210-567-3781
Email: TaylorAB@uthscsa.edu
Kristin Cano, Ph.D.
Technical Director
210-567-8706
Email: CanoK@uthscsa.edu
Eliza Ruben, Ph.D.
SBC HPC Systems Administrator
210-450-7050
Email: RubenE@uthscsa.edu
